Materials & Chemicals

Efficient Beta2-Amino Acid Synthesis Via Organocatalytic Aldehyde Aminomethylationlation
WARF: P06230US
Inventors: Samuel Gellman, Yonggui Chi, William Pomerantz, Emily English, William Horne, Li Guo
The Wisconsin Alumni Research Foundation (WARF) is seeking commercial partners interested in developing an efficient method for the synthesis of beta2 amino acids.
Overview
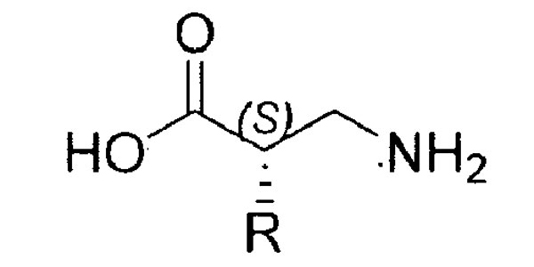
Beta amino acids are the basic building blocks of beta peptides, which have physiological applications, such as blocking human cytomegalovirus infection. Although some types of beta amino acids are readily available, beta2 amino acids, which are critical components of most beta-peptides, are difficult to synthesize and not commercially available.
The Invention
UW-Madison researchers have developed an efficient method for the synthesis of beta2 amino acids. The method is based on an asymmetric aldehyde aminomethylation that involves a Mannich reaction between an aldehyde, a formaldehyde-derived iminium ion, and an organic catalyst.
Applications
- Synthesis of alpha-substituted beta-amino aldehydes and beta-substituted gamma-amino alcohols
Key Benefits
- Overall yields greater than 40 percent, as compared to less than 10 percent for current synthesis methods
- Reduces the cost of producing beta2 amino acids
- Short, simple synthetic route with mild conditions
- Easily purified using minimal chromatography or recrystallization methods
- Uses commercially available, inexpensive starting materials
- Excellent stereoselectivity—can be diastereoselective or enantioselective
- Provides a wide array of naturally and non-naturally occurring side chains
- Easily scaled up to provide commercially useful yields
Additional Information
For More Information About the Inventors
For current licensing status, please contact Rafael Diaz at [javascript protected email address] or 608-960-9847
Figures