Pluripotent Stem Cells
Xeno-Free Protocol for Generating Endothelial Cells from Human Pluripotent Stem Cells
WARF: P140372US02
Inventors: James Thomson, Jue Zhang
The Wisconsin Alumni Research Foundation (WARF) is seeking commercial partners interested in developing the first fully defined method of its kind to derive human endothelial cells suitable for clinical applications.
Overview
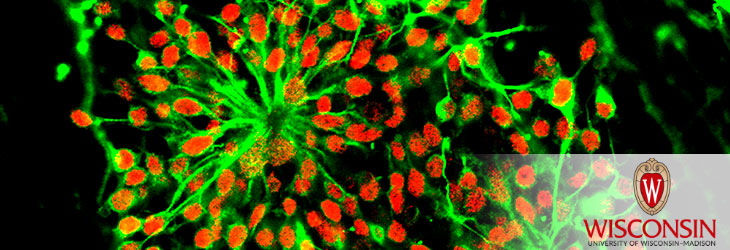
Endothelial cells (ECs) are vital to blood vessel formation. Generating ECs from human pluripotent stem cells (hPSCs) has powerful potential for cardiovascular health and drug development. However, standard protocols use xenogenic (animal-derived) reagents such as bovine serum albumin that are often poorly defined, pose safety concerns for clinical applications and contribute to highly variable results and limited reproducibility.
The Invention
UW–Madison researchers have developed a fully defined and xenogenic material-free method of producing and expanding clinically relevant human ECs for therapeutic and tissue modelling applications. Populations of up to 80 percent CD31+ ECs are generated from both human embryonic and induced pluripotent stem cells.
Like existing protocols, the new method uses factors including Bone Morphogenetic Protein (BMP), Activin A and a TGF-Beta 1 inhibitor.
Like existing protocols, the new method uses factors including Bone Morphogenetic Protein (BMP), Activin A and a TGF-Beta 1 inhibitor.
Applications
- Generation of clinical grade endothelial cells
- Potential applications include engineering of new blood vessels, EC transplantation into the heart, myocardial regeneration, treatment of regional ischemia, drug and toxicity screening
Key Benefits
- First xeno-free, albumin-free, fully chemically defined method of its kind
- Highly efficient and reproducible
Stage of Development
The protocol has been shown to generate 50-80 percent CD31+ ECs depending on the cell line. The results demonstrate robust and efficient generation of human ECs in fully defined conditions, which addresses a crucial production requirement for potential clinical utility.
Additional Information
For More Information About the Inventors
Related Technologies
Tech Fields
For current licensing status, please contact Andy DeTienne at [javascript protected email address] or 608-960-9857